How to Convert Alamar Blue Data to Proliferation Data
alamarBlue Protocols
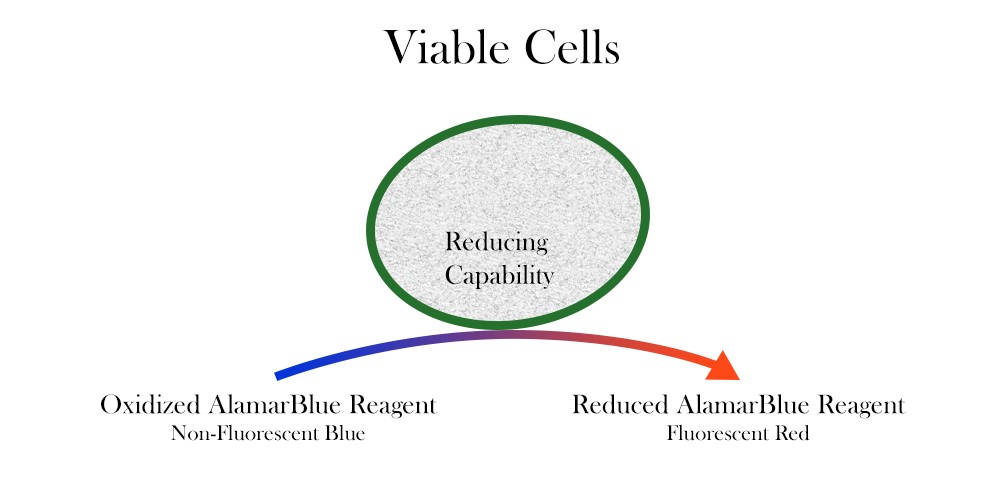
alamarBlue is a cell viability assay reagent which contains the cell permeable, non-toxic, and weakly fluorescent blue indicator dye resazurin. It has been extensively referenced and used in a wide range of research areas. alamarBlue quantitatively measures proliferation in human, animal, bacterial, fungal, and mycobacterial cells. It is useful for cytokine bioassays, cell viability assays, and in vitro cytotoxicity determinations as well as cell growth monitoring. Popular alamarBlue is a preferred alternative to other cell proliferation assays such as MTT, XTT, 3H Thymidine, or neutral red.
alamarBlue Work Principle
Resazurin is used as an oxidation-reduction (REDOX) indicator that undergoes colorimetric change in response to cellular metabolic reduction. The reduced form, resorufin, is pink and highly fluorescent, and the intensity of fluorescence produced is proportional to the number of living cells respiring. alamarBlue is a direct indicator of cell health and it detects the level of oxidation during respiration, quantitatively measuring cell viability and cytotoxicity.
Here are detailed protocols for use of alamarBlue.
- Quality Control Testing Method for alamarBlue
- AlamarBlue Cell Viability
- Determine LD50 Using alamarBlue
- Measuring Cytotoxicity or Proliferation Using alamarBlue
- Determining Optimum Length of Incubation and Plating Density
- alamarBlue Spectrophotometry Calculations with Different Filters
Quality Control Testing Method for alamarBlue
- Materials
- pH 7.4, 0.1M potassium phosphate buffer
- 10 mL test tube
- Pipettor capable of accurately dispensing 0.4 mL
- Plate reader with one of the following filters: 540, 570, 600, 630nm
- Dynatech flat bottom plate
- Method
- Pipette 0.4 ml of alamarBlue into a test tube.
- Dilute to 10 mL with phosphate buffer.
- Mix thoroughly.
- Pipette 100 µL into each well of a clear, flat bottom microblate.
- Read absorbance at appropriate wavelengths.
- Expected Results
| Wavelength (nm) | Average Absorbance (Standard Deviation) |
| 540 | 0.145 (0.002) |
| 570 | 0.225 (0.003) |
| 600 | 0.313 (0.004) |
| 630 | 0.116 (0.002) |
AlamarBlue Cell Viability
Protocol for assaying cell viability with a test compound:
- When cells are in log phase of growth, harvest them and determine the cell count.
- To appropriate wells of a 96 well plate, add the test compound and vehicle controls so that the final volume is 100µl in each well. Culture cells at 37°C in a cell culture incubator for desired test compound exposure period.
- Remove the assay plates from 37°C incubator and mix by gently shaking it.
- Aseptically add 10µl of alamarBlue Reagent in an amount equal to 10% of the volume in the well. In the Positive Control well, add 10µl of ultrapure sterile water. Incubate cultures at 37°C.
- After the incubation time ends, remove the plate and measure the fluorescence with Excitation wavelength at 530-560nm and Emission wavelength at 590nm.
Note: The suggested optimal cell count is 1 x 104 cells/ml. It may varybetween different cell lines.
Note: For positive control well, add 10µl of ultrapure sterile water.
Determine LD50 Using alamarBlue
alamarBlue can be used to determine the median lethal dose LD50 value and similar assays testing the effects of a toxin, radiation, or pathogen on cell cultures. Use semi-log graph paper to plot the percent of untreated control for each dilution of a given test agent on the y-axis vs. the concentration of the test agent on the x-axis. To determine the LD50 endpoint from the graph, read from where the 50% intercepts the dose.
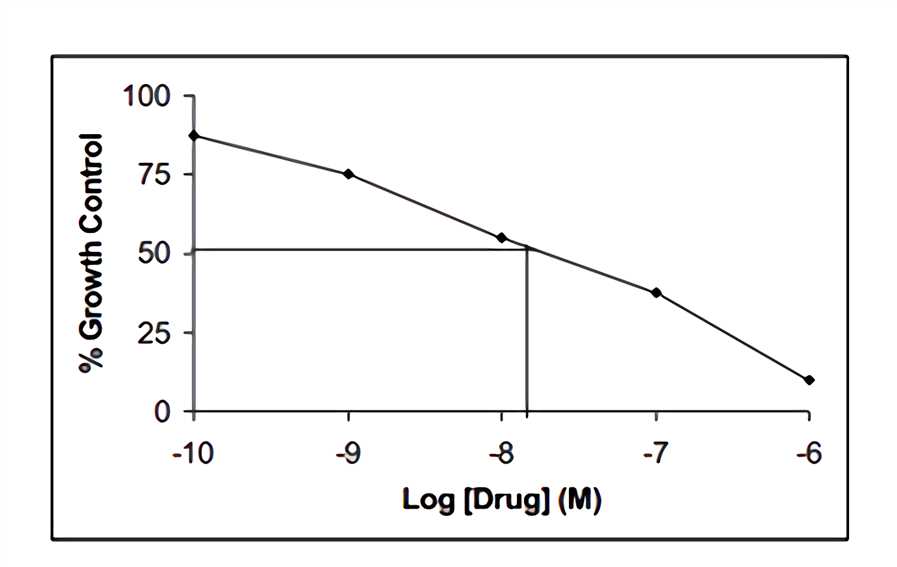 Fig.1 Response curve to the concentration along the x-axis.
Fig.1 Response curve to the concentration along the x-axis.
Measuring Cytotoxicity or Proliferation Using alamarBlue
- When cells are in log phase of growth, harvest them and determine the cell count.
- Plate cells and expose to test agent as determined by researcher. For determining the effect of a test agent on cell growth, ensure correct controls are included.
- Mix by shaking. Aseptically add alamarBlue in an amount equal to 10% of the volume in the well. Incubate cultures with alamarBlue for 4-8 h.
- Measure cytotoxicity or proliferation using spectrophotometry or fluorescence.
Note: The suggested optimal cell count is 1 x 104 cells/ml. It may varybetween different cell lines.
Note: The optimum incubation time may vary between cell types.
Determining Optimum Length of Incubation and Plating Density
The two variables which most affect the response of cells to alamarBlue are length of incubation time and number of cells plated. It is suggested that the plating density and incubation time should be determined for each cell line using the following steps.
- When cells are in log phase of growth, harvest them and determine the cell count.
- Plate cells at various densities, above and below the cell density expected to be used.
- Aseptically add alamarBlue in an amount equal to 10% of the volume in the well.
- Return plates to incubator. Remove the plate and measure fluorescence/absorbance each hour following plating for the first 6-8 h.
- For any given incubation time selected, the range in cell density relating cell number to alamarBlue reduction can be determined by a linear response.
- For any given cell density selected, the incubation time can be determined as the time taken for the control cells to turn the indicator from the oxidized (blue) form to the fully reduced (red) form.
- Measure absorbance at a wavelength of 570 nm and 600 nm; or measure fluorescence with an excitation wavelength at 530-560 nm and emission wavelength at 590 nm.
- Calculate the percentage reduction of alamarBlue at each cell density or incubation period.
Note: It is suggested that the plates remain in incubation overnight and measurements be made the following day at 24 h. Two kinds of information can be obtained from this data:
Percentage reduction of alamarBlue= [ (O2 x A1) - (O1 x A2) ] x 100%/ [ (R1 x N2) - (R2 x N1) ]
Where:
O1 = molar extinction coefficient (E) of oxidized alamarBlue (blue) at 570 nm
O2 = E of oxidized alamarBlue at 600 nm
R1 = E of reduced alamarBlue (red) at 570 nm
R2 = E of reduced alamarBlue at 600 nm
A1 = absorbance of test wells at 570 nm
A2 = absorbance of test wells at 600 nm
N1 = absorbance of negative control well (media plus alamarBlue but no cells) at 570 nm
N2 = absorbance of negative control well (media plus alamarBlue but no cells) at 600 nm
Only one appropriate substitute wavelength may be used.
alamarBlue Spectrophotometry Calculations with Different Filters
- Make up alamarBlue as directed in the package insert.
- Measure the absorbance of alamarBlue in media at the lower wavelength (LW) filter and at the higher wavelength (HW) filter.
- Measure the absorbance of 100 μL media only (blank) at the two wavelengths.
- Subtract the absorbance values of media only from the absorbance values of alamarBlue in media.
- Calculate correction factor (RO): RO=AO LW /AO HW
- Calculate percentage difference in reduction between treated and control cells in cytotoxicity/proliferation assays:
- If the alternative calculation to find the percentage reduction of alamarBlue is required, use the following equation:
Note: AOLW = absorbance of alamarBlue in media - absorbance of media only, lower wavelength
AOHW = absorbance of alamarBlue in media - absorbance of media only, higher wavelength
Percentage difference in reduction= [ (A LW - (AHW x Ro) for test well ] x 100%/ A LW - ( AHW x Ro ) for control well.
A LW = absorbance at lower wavelength minus the media blank
A HW = absorbance at higher wavelength minus the media blank
Ro = correction factor
Percentage reduction of alamarBlue = [A LW- ( AHW x Ro ) ] x 100%
Advantages of Using alamarBlue
- Reliable: Highly referenced for cytotoxicity and viability assays
- High Sensitivity: Detects as few as 50 cells
- Non-toxic: Does not kill cells and is safe to the user and the environment
- Simple: Water soluble, just add and measure the formula in cell cultures
- Flexible: Colorimetric and fluorometric detection. Suspended and attached cell lines
- Scalable: Effortless scale up for high throughput assays
- Stable: Proprietary buffering agents ensures alamarBlue is suitable for time course studies
- Economical: No cell lysis allows cells to be cultured for subsequent assays
Other Protocols
- Antibody Staining of Whole Mount Drosophila Embryos
- BrdU Protocol
- Primary Cultures for IHC-Viability Assays
- Sodium Azide Removal Protocol
- Biotin Conjugation
Source: https://www.antibody-creativebiolabs.com/alamarblue-protocols.htm
0 Response to "How to Convert Alamar Blue Data to Proliferation Data"
Post a Comment